
Introduction
Imatinib, approved as first-line treatment for patients with newly diagnosed chronic myeloid leukemia (CML) by FDA approximately 20 years ago, revolutionized the treatment of this disease. The life expectancy of newly diagnosed patients with CML has been approaching that of the global population. Second generation TKI (2GTKI) administered as a frontline therapy can induce deeper and faster molecular responses in a higher percentage of patients, however, the overall survival is comparable to that achieved with imatinib. To investigate the outcomes of long-lasting therapy with imatinib administered as initial therapy, we analyzed patients with CML who received imatinib as initial therapy at our institution starting from 2001.
Methods
We retrospectively analyzed long term outcomes of 267 patients treated with imatinib 400 mg at the Department of Hematology, Jagiellonian University Medical College, Cracow, Poland from 2001. Data from medical records were collected and statistical analysis was performed using R software (R version 4.0.2).
Results
The median age was 53.5 (16 to 88 years), 129 of patients (pts) (48.31%) were female. At the time of this analysis, 99 pts (37.08 %) remained on imatinib with the median dose at last follow-up (FU) 400 mg. The mean initial dose of imatinib was 410.4 mg/d. Imatinib dose was increased in 53 pts (19.85%), up to 800mg and up to 600 mg in 3 pts (1.13%), and in 49 pts (18.35%) respectively. The mean maximal dose was 465.2 mg. At baseline 124 pts (46.44%) had comorbidities: 79 pts (29.59%) vascular/cardiac, 11 pts (4.12%) renal, and 101 pts (37.33%) other comorbidities. 15 patients (5.63%) had prior malignancies, newly diagnosed malignancies occurred among 11 (4.12%) pts on imatinib. The median follow-up time was 11.37 years (range from 2 months to 19.5 years). 168 pts (62.92%) discontinued imatinib permanently, the median time to imatinib discontinuation was 2.02 years. Among them, 123 pts (71.93 %) switched imatinib to 2GTKI- 79 pts (29.59%) to dasatinib, 68 pts (25.47%) to nilotinib, and 14 pts (5.24%) to bosutinib. During the following treatment 87 pts (32,58%) received one 2GTKI, 33 pts (12.36%) two, and 3 pts (1.12%) more than two 2GTKIs. The main reasons for imatinib therapy discontinuation were intolerance (87 pts, 32.58%) and disease progression (90 pts, 33.71%). The median time to the imatinib discontinuation due to its intolerance was 2 years. Adverse events (AEs) during imatinib therapy were as follows: cardiac/vascular AEs in 22 pts (8.24%), renal in 42 pts (15.73%), hematologic in 43 pts (16.10%), and other in 189 pts (70.79%). Overall, 28 patients died (10.5%), 7 pts (2.2%) transformed to blast phase, 9 pts (3.37%) underwent allo-HSCT.
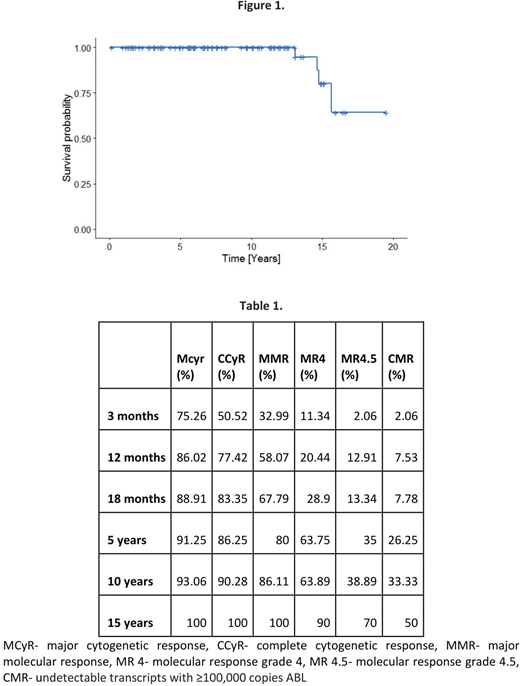
Estimated OS for patients that remained on imatinib for the whole observation period for 15 and 18 years was: 80.2%, and 64.1% respectively (Figure 1). Median follow-up time for patients who continued imatinib was 7.91 years. Intention to treat (ITT) analysis available for 99 pts (37.08%) revealed ITT responses at three months, one, five, ten and fifteen years: 50.52%, 77.4%, 86.25%, 90.28%, 100% for CCyR, 32.99%, 58.07%, 80%, 86.11%, 100% for MMR, 11.34%, 20.44%, 63.75%, 63.89%, 90% for MR4, 2.06%, 12.91%, 35%, 38.89%, 70% for MR4.5, 2.06%, 7.53%, 26.25%, 33.33%, 50% for CMR (undetectable transcripts with ≥100,000 copies ABL) (Table 1). The overall best response rates (at any time) for these 99 pts was 4.04% for MCyr, 5.05% for CCyR, 11.11% for MMR, 14.14% for MR4, 9.09% for MR4.5, 49.49% for CMR.
Conclusion
The analysis of long-term therapy with imatinib showed that the efficacy of imatinib persisted over time and that long-term administration of imatinib was associated with low rate of late toxic effects.
Sacha:Novartis: Consultancy, Honoraria, Speakers Bureau; Pfizer: Consultancy, Honoraria, Speakers Bureau; Adamed: Consultancy, Honoraria; Incyte: Consultancy, Honoraria, Speakers Bureau; Bristol-Myers Squibb Company: Consultancy, Honoraria, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal